Table of contents: O-ring for infusion pumps
- O-ring for infusion pumps
- Locations of O-rings in the pump unit
- Functional role and performance requirements per location
- Material selection
- Maintenance & replacement
- Inspection points & wear indicators
- Replacement interval, parts & validation
- FAQ: O-ring for infusion pumps
O-ring for infusion pumps
Locations of O-rings in the pump unit
Behind the front panel there are more seals than you might think at first glance. Think of the door/bezel assembly, the occluder and cam system, the motor and gearbox mount, and brackets for scanners or sensors. In all these subassemblies, the O-ring for infusion pumps prevents dust, moisture or cleaning agents from entering and stops mechanical tolerances drifting due to vibration. In some models a damping O-ring around the motor tames resonances; in others a small ring forms the edge seal between a cassette interface and the internal manifold. Such an o-ring for infusion pumps belongs to the infusion pump parts that are inspected periodically or replaced preventively.
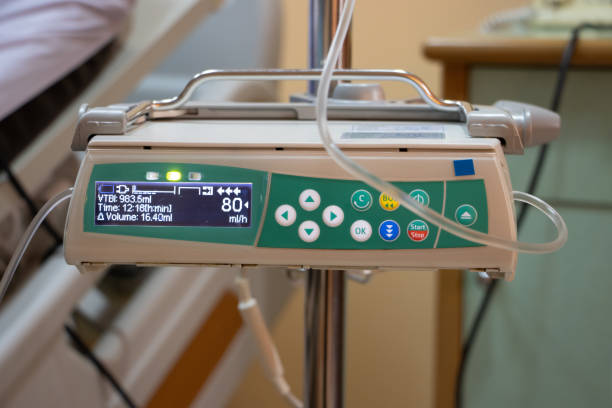
Functional role and performance requirements per location
Not every application requires the same specification. Around the door and the occluder mechanism, the O-ring for infusion pumps cushions and centers the moving part, so the line is reliably occluded and occlusion detection remains predictable. In a manifold or sensor interface, the same O-ring for infusion pumps ensures stable pressure transmission without micro-leakage that could trigger false alarms. At the motor mount and brackets, damping, positional stability and environmental sealing are key: the ring minimizes play, limits vibration and keeps cleaning fluids out. Thus the infusion-pump seal contributes to dosing accuracy, lower wear and fewer unplanned service stops.
Material selection
The right material selection for the O-ring for infusion pumps starts with environment and cleaning regime. For the internal O-ring for infusion pumps that regularly contacts cleaning agents or disinfectant sprays, Silicone is often the first candidate: elastic at low and high temperatures, chemically inert and available with USP Class VI for medical applications. In areas with a lot of ozone, steam or oxidizing cleaners, EPDM offers an excellent combination of ageing resistance and low compression set. For locations that see heat and more aggressive chemicals, for example around motor cooling or solvent contact, FKM is a logical step. Whatever route you choose: ensure biocompatibility where needed, record compound codes and link each ring to a clear bill of materials.
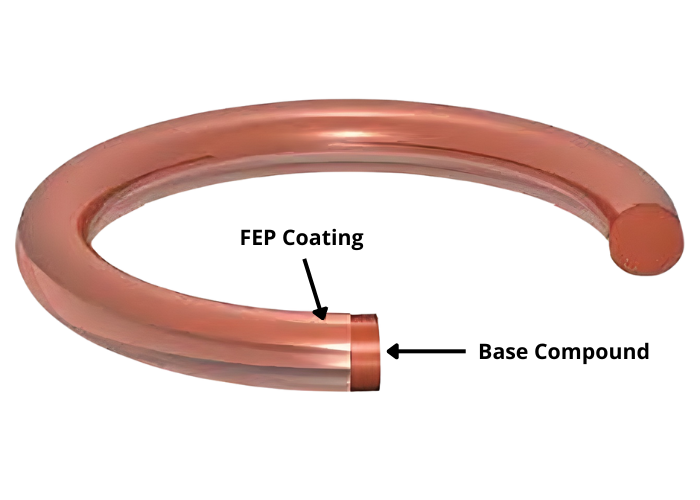
For extreme chemical resistance or minimal stick–slip, an FEP-encapsulated O-ring can be the answer. This combines an elastomeric core (resilience, sealing force) with a seamless fluoropolymer jacket (chemical resistance, low friction). Note: the stiffer jacket requires neat groove finish and correct compression, otherwise the restoring force drops. Even within standard elastomers, fine-tuning remains needed: hardness (Shore A) for shape retention, surface finish for low friction, and batch traceability for reproducible performance. This way you benefit maximally from the O-ring for infusion pumps without unwanted variation between units. And by validating test pieces with the supplier in time, you reduce the chance of surprises in series production with an O-ring for infusion pumps.
Maintenance & replacement
Inspection points & wear indicators
Preventive maintenance is about looking, measuring and documenting. During services, check the elastic response, roundness and any flat spots (flattened cross-section). An O-ring for infusion pumps showing early cracking, glossy patches, increased hardness or clear imprints from burrs in the groove indicates replacement. Always clean according to the method sheet: incorrect solvents can accelerate premature ageing, even with FKM or EPDM. Tip: record per position which compound and hardness are installed and link findings to fault codes. This way an O-ring for infusion pumps is not "just" a ring, but a controlled component with a predictable service life.
Replacement interval, parts & validation
Plan intervals based on environment (temperature, chemicals), load (number of cycles) and service data. Combine replacement with a short validation: leak/pressure test where applicable, and a functional check of occlusion and air alarms. Keep a minimal set of infusion-pump parts in stock: rings per position, the right hardness, any assembly grease compatible with your compound, and clear labels per batch. Document batch numbers and set up a cross-reference to the pump’s serial number. This turns "parts swapping" into a repeatable process with demonstrable quality, and reduces the chance that a freshly replaced O-ring for infusion pumps needs attention again too soon due to a selection or assembly error.
FAQ: O-ring for infusion pumps
Not in standard IV sets: they rely on welded/clamped parts and the Luer cone. The O-rings are in the pump unit for environmental and mechanical sealing, not in the patient fluid path.
For an O-ring for infusion pumps, silicone is popular for its elasticity and inertia; EPDM excels with water/ozone and many cleaning cycles; FKM performs well under heat and chemical load; and an FEP-encapsulated O-ring combines chemical resistance with resilience. Request USP Class VI where needed to cover biocompatibility.
Watch for loss of resilience, cracks, glossy spots from friction, surface erosion, or a noticeably flattened cross-section. These are classic replacement triggers.
Only if allowed by the supplier and with a lubricant compatible with your compound and application. Over-application can trap dirt or affect measurements.
Define the function and environment per position, choose the material profile (Silicone, EPDM, FKM or FEP-encapsulated), request USP Class VI where relevant, define hardness and dimensions, and validate the set-up in your own cleaning and service protocol.


 Nederlands
Nederlands  Deutsch
Deutsch  Français
Français  Italiano
Italiano  Español
Español 